
Edition 5 - April 1998
 Edition 5 - April 1998 |
The Budapest Treaty:
|
 Cryopreservation of patented cultures in liquid nitrogen. |
One requirement of patent law is that all the details of an
invention must be publicly disclosed. For disclosure to be
adequate, an invention must be described in sufficient detail to
permit a person skilled in the art to reproduce it.
Disclosure normally takes the form of a written description and drawings. However, inventions involving the use of microorganisms or other biological materials present problems, since repeatability often cannot be ensured by means of a written description alone. As a result, it was agreed that the deposit of the involved biological material concerned in an officially recognised culture collection was to be part of the patenting procedure. This led to the need for a uniform international system, defining the minimum criteria to be met by receptory collections and avoiding the need to deposit the same organism in different countries.
As such, the "Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure" was concluded in 1977 under the auspices of the World Intellectual Property Organisation (WIPO) and came into force in 1980. To date, 43 States and one Intergovernmental Industrial Property Organisation (the European Patent Office) have ratified the Treaty.
The fundamental principle of the Budapest Treaty is that all States party to it consider a deposit in just one IDA as sufficient for their patent procedure.
A culture collection can become an IDA if it has been formally nominated by its host State and when that State has furnished solemn assurances that the collection complies and will continue to comply with the requirements of the Treaty and Regulations. Worldwide, 30 culture collections, including the BCCMTM (see box below) have obtained IDA status.
The Regulations under the Treaty stipulate that the IDA must be available on the same terms to any depositor. It must accept and store the microorganisms deposited with it and furnish samples only to those entitled to receive them. Provisions are made to guard against the loss and consequent non-availability of deposited microorganisms. An IDA must have the expertise and facilities necessary to keep the microorganism viable and uncontaminated throughout the storage period required by the Treaty (a minimum of 30 years).
Remarkably, the term "microorganism" is not defined in the Budapest Treaty and jurisprudence demonstrates its interpretation in a broad sense. Whether an entity is technically a microorganism matters less in practice than whether deposit of that entity is necessary for the purposes of disclosure. Thus, for example, tissue cultures and plasmids can be deposited under the terms of the Treaty, even though they are not microorganisms in the strict sense.
Contact
M. BOSSCHAERTS, Quality & Regulatory affairs manager
TEL: +32 2 238 36 07
FAX: +32 2 230 59 12
E-MAIL: BOSC@BELSPO.BE
International Depositary Authorities (IDAs) can, on a voluntary basis, adhere to a Code of Practice for IDAs, which provides guidelines on how to deal with some situations in the patent deposit procedure.
The Budapest Treaty (BT) is a good basis for the work and responsibilities of culture collections with IDA status.
Nevertheless, at the annual meeting of the European Culture Collections' Organisation (ECCO) in Slovenia (July 3-4, 1995), it was felt that the Treaty does not always give explicit solutions to all issues encountered by IDAs and that, in reality, each IDA has its own procedures.
To harmonise different approaches, it was decided that an inventory of these practices should be drawn up.
After consultation with the European IDAs, the initiative was broadened to include non-European IDAs. Furthermore, the World Intellectual Property Organisation (WIPO, Geneva) and the European Patent Office (EPO, Munich) were informed of the initiative and provided valuable input.
The resulting Code of Practice summarises the points where consensus among IDAs exists, and provides guidelines on how to deal with unclear situations in the patent deposit procedure. It is a tool for assuring that all IDAs apply the same principles to handling deposits. It is flexible, enabling an IDA to impose additional requirements to comply with internal or national regulations, but supports the intent of the Treaty to harmonise the deposit requirement, which should ultimately also be in the advantage of depositors.
Besides a short summary of the obligations of both the depositor and the IDA, the document describes situations that can be encountered by an IDA and shows how to react on them.
For example, when a depositor does not provide all the necessary cultivation, storage and viability testing information for the deposited organism, the Code of Practice admits that an IDA can continue the deposit procedure applying its own standard methods for preservation and cultivation. If necessary, the IDA should agree methods with the depositor.
Although the date of deposit remains the date of receipt of
the viable biological material, the deposit will only be accepted
when the depositor has complied with all his obligations. To
avoid delays, depositors are advised to provide the IDA with the
mandatory information at the outset.
 |
It was also agreed that, if a deposit is made with an IDA outside the purview of the Treaty (e.g. safe or public deposits, or deposits formerly made under national patent law), a depositor can subsequently convert such a deposit to a deposit under the BT without depositing the material again. The date of deposit remains the date on which the IDA received the viable organism. It is, however, essential that the deposit is under the regime of the BT and that all the requirements are met, at the latest, on the filing date of the patent application. In cases of conversion, both the date of deposit and the date of receipt of the request for conversion must be stated on the "Receipt and acceptance" form BP/4. The legal consequences of the deposit during the period between the two dates is determined by national law.
Although, in principle, most IDAs accept mixed cultures, they generally advise the depositor to separate the components and to deposit them separately. In this way, problems related to antagonism and different growth rates of the components are avoided.
If an IDA receives a contaminated culture, it should ask the depositor to send another, pure culture. Here, the deposit date will be the date on which the IDA receives this pure culture. Alternatively - and to keep the original deposit date - the IDA can offer to purify the culture. To be sure that the correct culture is deposited, the IDA should then send a sample of the purified and preserved culture to the depositor, to check its authenticity.
The Code of Practice confirms that parties entitled to receive a sample of a deposited organism should be provided with the minimum information necessary to enable them to correctly handle and analyse it. No further information should be available to third parties.
The Code of Practice also deals with matters such as the payment or withdrawal of a deposit and the responsibility for authenticity and purity of the cultures. Finally, a list of test methods and criteria for viability testing of different types of organisms is included.
BCCMTM has recentely sent the Code of Practice to all 30 IDAs to ask their formal agreement with the described principles. Yet, most of them have reacted positively. Full text of the Code of Practice can be consulted on the BCCMTM home page.
Contact
M. Bosschaerts, Quality & Regulatory affairs manager
TEL: +32 2 238 36 07
FAX: +32 2 230 59 12
E-MAIL: BOSC@BELSPO.BE
Prof. Thierry Bogaert is the dynamic founder of a functional genomics and drug discovery company called DevGen. The company enhances and accelerates the drug discovery process using the tiny worm Caenorhabditis elegans. In order to protect DevGen's research results, Prof. Bogaert has deposited genetically modified forms of this nematode with the BCCMTM/LMBP. BCCMTM News talked to Prof. Bogaert and Mrs Vanhoucke, curator of the BCCMTM/LMBP plasmid collection.
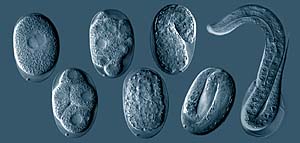 BCCMTM/LMBP has accepted genetically modified forms of the nematode C. elegans as patent deposits under the Budapest Treaty. |
BCCMTM News: Prof. Bogaert,
what kind of organism is C. elegans and why is it so interesting
to be investigated?
Prof. Bogaert: C. elegans is the best characterised
multicellular eukaryote. Its morphology and development, and the
function of each of its cells, have been mapped in exquisite
detail. In addition, these nematodes are transparent, permitting
full in vivo visualisation of the most subtle cellular
phenotypes. In the academic world C. elegans is well known as
a standard tool for the study of animal developmental and cell
biology.
It is an interesting organism because essentially all the genes
and pathways shown to be important in cell-, developmental- and
disease-biology are now known to be conserved between worm and
man. Biological information gained from the nematode can be
used to unravel human disease.
What role do plasmids play in your research?
We inject plasmid DNA carrying a human gene of interest into the
gonad of specific deletion mutants of the worm. After cell
division, some of the cells will contain the plasmid DNA as a
kind of "mini-chromosome". By comparing the phenotypes
of the mutants and those of the plasmid-carrying organisms, C.
elegans can be used to discover molecular switches that, when
triggered, selectively alleviate disease. It can also identify
lead therapeutic compounds acting on these switches.
Why did you decide to deposit these cultures under the
Budapest Treaty?
Like transgenic mice, these plasmid-carrying nematodes are animal
disease models that can be used to discover drugs and drug
targets. I patented these organisms to gain international
protection of my research results through one single patent
deposit with one professional IDA. Under patent law, this
enables every professional "skilled in the art" to
repeat our experiments.
Mrs Vanhoucke, could the BCCMTM/LMBP
accept this kind of biological material?
Mrs Vanhoucke: According to the notification made by the Belgian
government to the Director General of WIPO, the BCCMTM/LMBP
collection must accept, among others, "genetic material,
whether recombinant or not - such as plasmids, oncogenes and RNA
- as isolated material preparations or in a host". While
at the BCCMTM/LMBP the usual hosts for plasmids are E.
coli strains, Prof. Bogaert's somewhat peculiar request still
fitted within this definition, and within our technical
capacities. We were therefore able to accept the proposed
deposits.
The BCCMTM Consortium as an IDASince March 1, 1992, the BCCMTM
consortium has been recognised as an "International
Depositary Authority" (IDA) by the World
Intellectual Property Organisation (WIPO). Therefore, the
BCCMTM collections can accept as patent
deposits under the Budapest Treaty: In fact, BCCMTM accepts almost all types of biological material except plant seeds and plant tissue cultures. Patented strains are preserved separately from the public collection. Access to this material and related information is rigorously controlled. The use of multiple state-of-the-art preservation methods (e.g. freeze drying and cryopreservation over liquid N2), together with storage of duplicates at a second location, guarantees a very high degree of security. To support potential depositors, the BCCMTM has produced a practical manual, which clearly describes the chronological steps in the deposit procedure. This manual is available free of charge from the BCCMTM on request, and can also be consulted on line (http://www.belspo.be/bccm/tbu). The Budapest Treaty strongly advises the depositor to mention the scientific description and/or proposed taxonomic designation of the deposited organism. The BCCMTM collections have built up substantial expertise in techniques for molecular fingerprinting and can assist the depositor in making the scientific description and taxonomic designation. A broad range of identification and characterisation methods (AFLP™, PFGE, fatty acid profiling, etc.) is available. Moreover, the IDA status of the BCCMTM allows its collections to accept safe deposits for other legal purposes such as, for example, the animal feed directive (2). Safe deposits are never catalogued and subcultures are only made available to the depositor or an authorised mandatary. Moreover, they can be converted to patent deposits at any time. (1) see case study. |
Home |
Contents Edition 5 - April 1998 |
Next Article Edition 5 - April 1998 |